18 Aug Expanded Access vs. Right to Try: What Patients Need to Know
What is the Food & Drug Administration’s (FDA) Expanded Access Program?
Also known as “compassionate use,” this program allows people with immediately life-threatening conditions or serious diseases or conditions to gain access to investigational medical products. According to the FDA, an investigational medical product is a new drug, biologic, or medical device that is still under clinical investigation and has not yet been finally approved for use by the FDA.
If you are interested in getting access to a treatment option through the FDA’s Expanded Access program, your chances of success are high! One study conducted over a 10-year period (January 2005 to December 2014) by Dr. Steven Joffe from the University of Pennsylvania’s Perelman School of Medicine found that the FDA approved 99% of all expanded access requests for almost 9,000 investigational drugs.
To apply for Expanded Access:
-
-
- The patient consults with their licensed physician to make sure they have exhausted all other treatment options.
- The licensed physician oversees the patient’s treatment and reaches out to the medical product developer, files paperwork with FDA and Institutional Review Board, and takes charge of patient care and reporting.
- The industry (product developer) agrees to provide the investigational medical product and submits the expanded access request to the FDA, or uses another avenue to submit the expanded access request.
- The Institutional Review Board (IRB) reviews the expanded access protocol and consent forms to make sure the patient understands the nature of the treatment.
- The FDA reviews the expanded access protocol and determines if the treatment can proceed.
-
What is the Right to Try Act?
The Right to Try Act, or the Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act, was signed into law on May 30, 2018. Before this federal law, 41 states including California, Florida, and Pennsylvania already had state “right to try” laws in place. Make sure to check Triage Cancer’s chart on cancer-related state laws for more information on state-specific right to try legislation.
Similar to the Expanded Access Program, the Right to Try Act allows patients with serious or immediately life-threatening diseases or conditions who are unable to participate in clinical trials to access certain unapproved treatments.
To apply for treatment under the Right to Try Act:
-
-
- The patient speaks to their physician, who will contact the manufacturer of an investigational treatment in an FDA-approved Phase 2 or 3 trial.
- The manufacturer agrees, and together with the patient’s doctor, develops a treatment protocol specific to patient.
- The manufacturer will provide patient with the cost if cost-recovery is necessary (manufacturers may not profit from unapproved drugs).
- The patient signs state-compliant informed consent document.
- The manufacturer notifies the FDA within a year that the treatment took place and reports outcome data.
-
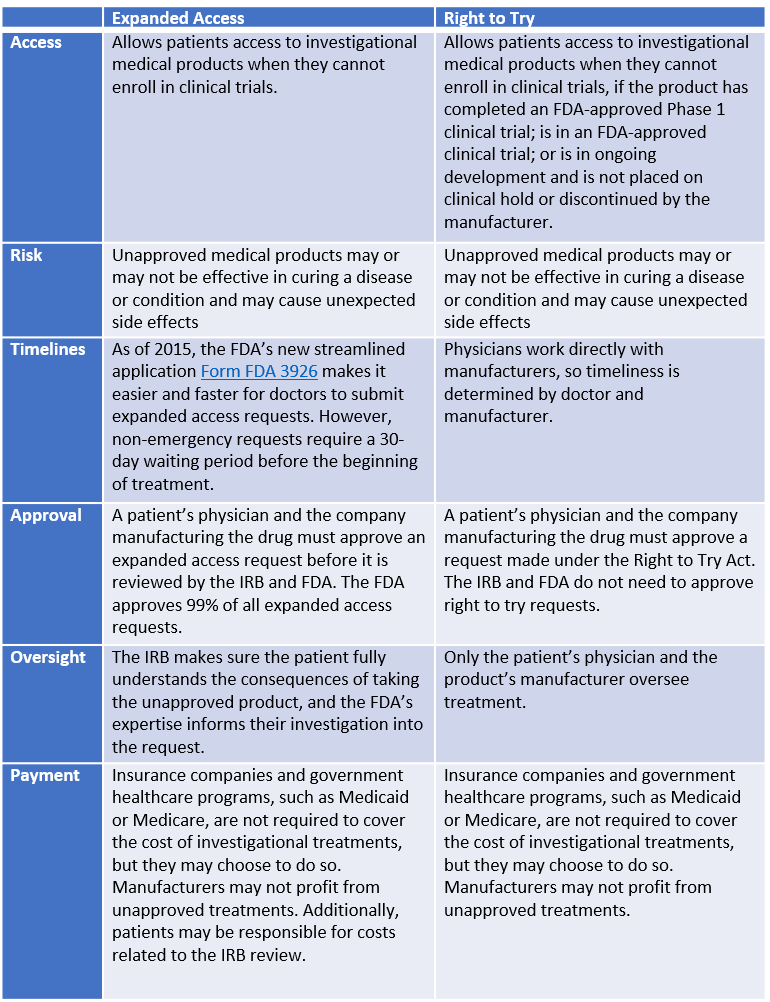
FDA’s Expanded Access Program vs. Federal Right to Try Act
For more information, read:
- FDA’s Guide to Expanded Access
- FDA’s Guide to Submitting Expanded Access Requests
- FDA’s Fact Sheet on Right to Try Law
For more information about clinical trials, visit http://TriageCancer.org/ClinicalTrials and www.CancerFinances.org.